We had plates with starch agar, and plates with starch agar + GA. Seed halves (with and without embryo) were assigned to quadrants in each plate. (It's important to note that when we split our barley seeds in half, the embryo half would still have some endosperm/starch, as seen above, and would also retain some of the aleurone layer -- we didn't perfectly isolate the embryo.) We then stained the plates with iodine to look for presence of starch hydrolysis -- which would show up as "halos" (see below) of light-colored agar where starch had been hydrolyzed into glucose molecules (which do not stain black with iodine like starch does).
Starch + GA plate with "hydrolysis halos"
We also looked at the GA-->hydrolysis pathway from another angle -- accumulation of glucose (as opposed to absence of starch).
We did this with barley seed halves as before, but then we tested for presence of glucose using Clinistix test strips.
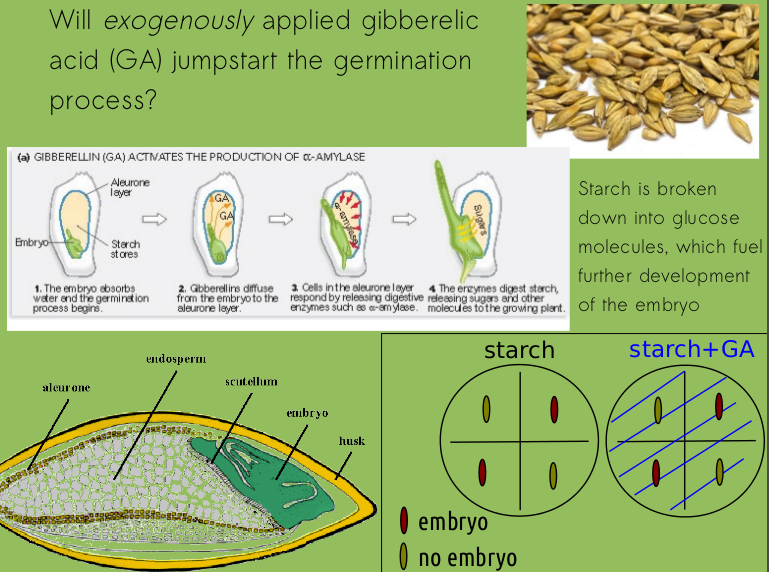
So what would be our expected results from these experiments? Something like this, right?
Without the embryo to release the signal molecule GA, embryo-less seed halves on the starch plate would have no "trigger" to initiate production of alpha-amylase, the enzyme that facilitates starch hydrolysis. But when we provide that hormone on the starch + GA plate, even embryo-less barley halves should show signs of hydrolysis. (We'll just worry about presence/absence of hydrolysis now, not relative intensities or halo size.)
Same idea here: without GA, the embryo-less seed halves won't hydrolyze any starch (thus, there shouldn't be any significant level of glucose when we test for it).
But this isn't what we saw in lab, was it? No! We saw (with a few "correct" exceptions) high glucose levels in all tubes, and hydrolysis halos in all treatments! What's up with that??
Of course we could have mixed up our seed halves, right? That hilum can be hard to distinguish sometimes. But many of the seed halves on the agar plates had begun to germinate, allowing us to double-check ourselves -- and all the ones I checked were correct (also, the Tuesday labs saw similar results). Perhaps some groups, when cutting their seeds, included a small portion of the scutellum (where the GA is released from), in their embryo-less halves. Contamination or mixing of plates/tubes could also be an issue, but likely it has more to do with processes going on in the seeds themselves. Most importantly, it's likely that some early germination processes had been initiated in many of the barley seeds, perhaps by storage in a humid environment, so some amount of GA had actually already been sent to the aleurone layer, promoting the production of alpha-amylase and beginning the starch hydrolysis process in the endosperm. Thus, even in our embryo-less / endosperm halves, we were seeing evidence of alpha-amylase activity through starch reduction and glucose accumulation.
Here we just looked at presence/absence of starch hydrolysis. What hypotheses would you make about relative intensities of this process among the different treatments (we talked about this a bit in class)? i.e., which halos would be bigger? where would we find more or less glucose?